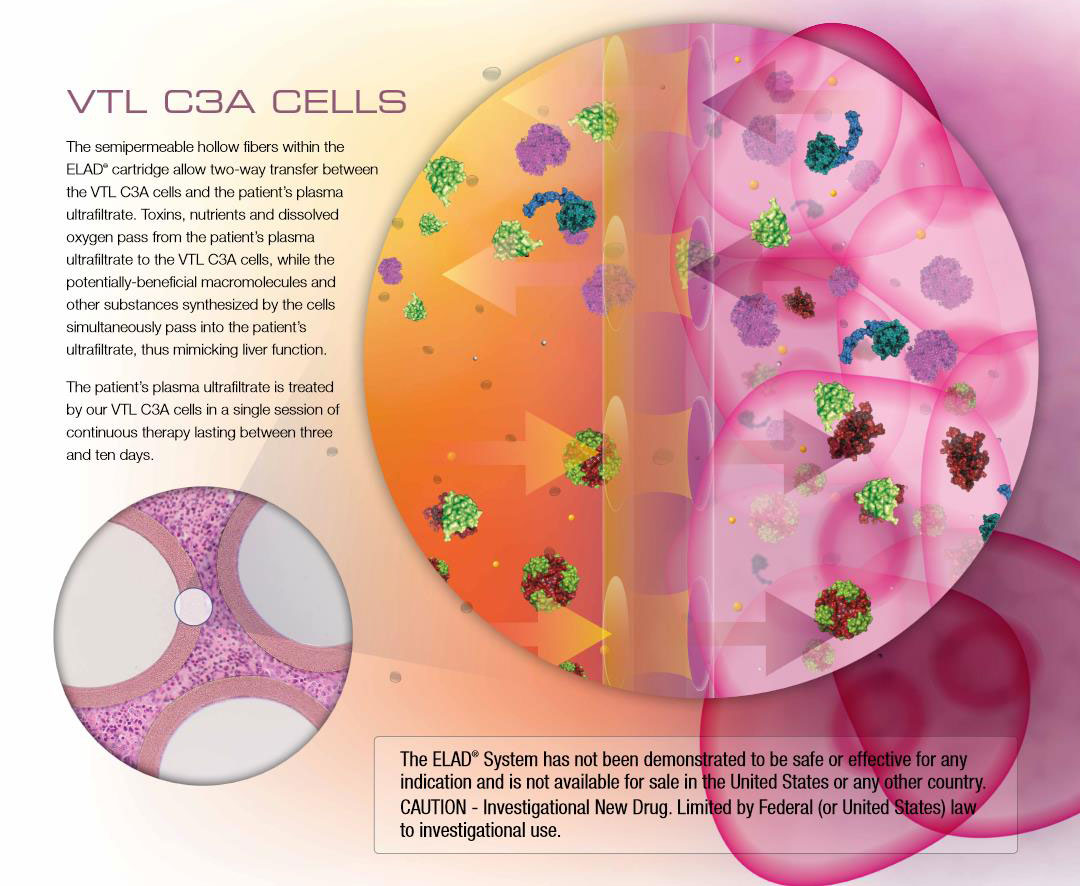
SD ELAD Biotechnology Co. Ltd., is developing the world first human liver cell-based therapy that provides metabolic support for patients with severe liver failure, the Extracorporeal Liver Assist Device (ELAD®). ELAD bioartificial liver therapy is based on the human hepatoblastoma-derived, immortalized cell line C3A, cultured in hollow fiber cartridges. The cells are the key in supporting a liver failure patient by processing liver toxins and synthesizing human liver proteins. The basic function of ELAD is after 5-10 days continuous treatment, to let the patient own liver function completely recover and to have the normal person’s life, or to give the critical patient enough time to find a suitable donor liver, and successfully bridge to liver transplantation. To date, 627 patients have participated in ten ELAD clinical trials in the United States (US), United Kingdom, European Union, China and Singapore.
The technology of the ELAD cell bioreactor and clinical ELAD System is now owned by SD ELAD Biotechnology Co. Ltd., a Sino-China joint venture. This technology has been developed in the US for thirty years by Vital Therapies, Inc. (VTL) and two predecessor companies, Hepatix Inc. and Vitagen Inc. More than 500 million USD has been invested in the development of ELAD. SD ELAD Biotechnology is headquartered in Jinan high tech zone, Shandong province, China, and the ELAD product will be manufactured at the same location.
In 2019, SD ELAD Biotechnology Co. Ltd., purchased all assets related to ELAD from VTL. Vital Therapies finished a pivotal, phase 3 clinical trial in severe alcoholic hepatitis in US/EU in 2018. Although there was a numerical improvement in survival in the ELAD-treated group between three months and one year following randomization, the study failed to meet the primary endpoint of a significant improvement in overall survival through at least ninety-one days assessed using the Kaplan-Meier statistical method.
Furthermore, the most prevalent Treatment Emergent Adverse Events (TEAEs or TESAEs) related to ELAD treatment appeared to be anemia, coagulopathy, hypotension, and thrombocytopenia; a safety profile that has been consistently observed in the prior ELAD studies. These types of TEAEs or TESAEs are frequently observed with other types of extracorporeal therapy, are therefore expected, and are not unique to treatment with ELAD. These findings suggest that further evaluation of ELAD in a different patient population would not result in an unfavorable risk/benefit profile.
In light of trial results, VTL did not believe the ELAD System could not be approved in the United States or European Union without additional clinical trials that would require substantial amount of capital and time to complete. Consequently, the VTL ceased any further development of ELAD and sold the assets and rights to SD ELAD Biotechnology. VTL later merged with Immunic, Inc., a private German company pursuing a completely separate technology, and is now known as Immunic Therapeutics (Immunic Inc. [Nasdaq: IMUX]).
SD ELAD Biotechnology feels a strong sense of urgency to reach out to the thousands of liver failure patients in China with ELAD therapy. In China approximately 400,000 patients die every year due to liver failure caused by hepatitis B/C/E, drugs and toxins, vascular problems, metabolic imbalance, surgery, autoimmune and other unknown reasons. Liver failure is the fourth leading cause of death in China. China now has almost 100 million carriers of hepatitis viruses. Each year about 2 million Chinese suffer from severe liver failure, while there were about 2,896 liver transplants performed in 2005. By 2017, after 12 years, only 4,732 liver transplants were performed in China.1
Vital Therapies conducted a clinical trial with ELAD in China for acute flares of Hepatitis B (acute-on-chronic) in 2006-07. Two infectious disease hospitals in Beijing, Capital Medical University, YouAn Hospital and PLA 302 Hospital, were chosen for the study. The ELAD clinical trial in China was designed with expected mortality in the standard of care (control) group of 50%. Kaplan-Meier Plot of Transplant-Free Survival (TPS) showed ELAD treatment significantly improved the survival rate of the enrolled acute-on-chronic liver failure patients with the 28- and 56-day transplant-free survival rates reaching 88% and 80%, respectively. The control group had 28- and 56-day transplant-free survival rates of 50% and 44%, respectively.
A statistically significantly higher proportion of transplant-free survivors in the ELAD group vs Control group was observed at day 28 (p=0.015) and day 56 (p=0.018).2 The overall survival through 90 days demonstrated significant improvement in ELAD treated patients’ survival compared to Controls (p=0.046, Wilcoxon); Three years after treatment ELAD vs Control showed p=0.045 (Log Rank), p=0.041 (Wilcoxon).
In the United States, an open Investigational New Drug (IND) file continues to be maintained with the US Food & Drug Administration (FDA). The serial amendments to the IND by SD ELAD Biotechnology and all predecessors track the development and clinical studies involving ELAD technology. Similarly, all materials submitted to the various regulatory authorities in the European Union (EU) are retained by SD ELAD Biotech. SD ELAD Biotechnology will maintain contact and communication with US FDA and European Medicines Agency (EMA)
Address: floor 1, block B (21b), neijuxuan workshop, comprehensive bonded zone, No. 33688, jingshidong Road, Jinan area, China (Shandong) pilot Free Trade Zone Telephone:
Copyright: Shandong yiruite Biotechnology Co., Ltd Record No:鲁ICP备20021086 Sign in